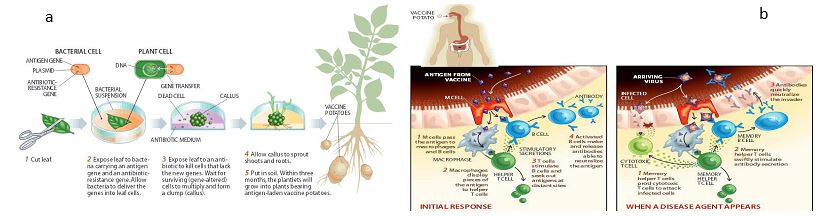
Edible vaccines are less expensive, easily administrable, storable, and are widely accepted as bio-friendly, particularly in developing countries. Oral administration of edible vaccines appears to be a promising agent for reducing the incidence of various diseases such as hepatitis and diarrhea, where vaccine preservation and management problem. Edible vaccines are obtained by incorporating a particular gene of interest into the plant, which produces the desirable encoded protein (Langridge, 2000). Cereals (wheat, rice, corn), fruits (bananas), and vegetables (lettuce, potatoes, tomatoes) are among the foods utilized as an alternative agent for injectable vaccinations. There are two approaches to treating the selected gene that was extracted from the bacteria that encode a certain antigen:
- The desired peptides/proteins are genetically modified into a suitable plant virus. The recombinant virus is then incorporated into the plant, which enables it to produce a huge number of new plants from which chimeric virions are isolated and purified. The consequential edible plant vaccine can then be used for immunological applications.
- Another technique involves incorporating the desired gene into the plant vector via transformation. Many alternative ways have been used, which may be classified as follows: Agrobacterium-mediated gene transfer, Biolistic method, and Electroporation.
All human pathogens enter the body through mucosal surfaces, which include the urogenital, respiratory, and gastrointestinal systems. The most efficient path of mucosal immunization is the oral route because oral vaccines can produce mucosal immunity, antibody-mediated immune response, and cell-mediated immune response (Arakawa et al. 1998). As an advantage orally, administered antigen-containing plant vaccines do not get hydrolyzed by gastric enzymes due to the tough outer wall of the plant cell. Transgenic plants containing antigens act by the process of bio-encapsulation, i.e., outer rigid cell wall, and are finally hydrolyzed and released in the intestines. The released antigens are taken up by M cells in the intestinal lining that is placed on Payer’s patches and gut-associated lymphoid tissue (GALT). These are further passed on to macrophages and local lymphocyte populations, producing serum IgG, IgE responses, local IgA responses, and memory cells, that rapidly counterbalance the attack by the real infectious agent. Edible vaccinations are generally accepted because, unlike typical injectable immunizations, they are delivered orally. As a result, they do not require skilled medical personnel, and the risk of contamination is decreased because they do not require sterilization of the premises and production area. Some major problems of edible vaccines are: edible vaccines rely on plant stability since many plants, such as potatoes, cannot be consumed raw and must be cooked, which causes denaturation or weakening of the protein present and are prone to get microbial infestation e.g. potatoes containing vaccine can last long if stored at 4°C while a tomato cannot last long (Moss et al.1999).
Dr. Md. Monirul Islam
Senior Scientist
ASRBC, ACI Seed
References:
- Arakawa T, Yu J, Chong DK, Hough J, Engen PC, Langridge WH. A plant-based cholera toxin B subunit-insulin fusion protein protects against the development of autoimmune diabetes. Nat. Biotechnol. 1998: 16; 934-938.
- Jan N, Shafi F, bin Hameed O, Muzaffar K, Dar SM, Majid I, et al. An Overview on Edible Vaccines and Immunization. Austin J. Nutri. Food Sci. 2016; 4(2): 1078.
- Langridge WH. Edible vaccines. Sci Am. 2000; 283: 66-71.
- Moss WJ, Cutts F, Griffin DE. Implications of the human immunodeficiency virus epidemic for control and eradication of measles. Clin Infect Dis. 1999; 29: 106-112.